_Hydrothermal Salt Theory
Abstract AGU, 2010 Fall meeting
Session: EP01 Earth and Planetary Surface Processes General Contributions
Session: EP01 Earth and Planetary Surface Processes General Contributions
A universal salt model based on under-ground precipitation of solid salts due to supercritical water ‘out-salting’
|
Martin Hovland, Centre for Geobiology, University of Bergen, Bergen, Norway, and Statoil ASA, Stavanger, Norway
Håkon Rueslåtten, NumericalRocks ASA, Trondheim, Norway Hans Konrad Johnsen, Det Norske ASA, Trondheim, Norway Tore Indreiten, Statoil ASA, Stavanger, Norway Abstract One of the common characteristics of planets Earth and Mars is that both host water (H2O) and large accumulations of salt. Whereas Earth’s surface-environment can be regarded as ‘water-friendly’ and ‘salt hostile’, the reverse can be said for the surface of Mars. This is because liquid water is stable on Earth, and the atmosphere transports humidity around the globe, whereas on planet Mars, liquid water is unstable, rendering the atmosphere dry and, therefore, ‘salt-friendly’. The riddle as to how the salt accumulated in various locations on those two planets, is one of long-lasting and great debate. The salt accumulations on Earth are traditionally termed ‘evaporites’, meaning that they formed as a consequence of the evaporation of large masses of seawater. How the accumulations on Mars formed is much harder to explain, as an ocean only existed briefly. Although water molecules and OH-groups may exist in abundance in bound form (crystal water, adsorbed water, etc.), the only place where free water is expected to be stable on Mars is within underground faults, fractures, and crevices. Here it likely occurs as brine or in the form of ice. |
Based on these conditions, a key to understanding the accumulation of large deposits of salt on both planets is linked to how brines behave in the subsurface when pressurized and heated beyond their supercritical point. At depths greater than about 3 km (P>300 bars) water will no longer boil in a steam phase. Rather, it becomes supercritical and will attain the phase of supercritical water vapor (SCRIW) with a specific gravity of typically 0.3 g/cm3. An important characteristic of SCRIW is its inability to dissolve the common sea salts. The salt dissolved in the brines will therefore precipitate as solid particles when brines (seawater on the Earth) move into the supercritical P&T-domain (above 400ºC and 300 bars).
Numerical modeling of a hydrothermal system in the Atlantis II Deep of the Red Sea indicates that a shallow magma-chamber causes a sufficiently high heat-flow to drive a convection cell of seawater. The model shows that salt precipitates along the flow lines within the supercritical region (Hovland et al., 2006). During the various stages of planet Mars’ development, it must be inferred that zones with very high heat-flow also existed there. This meant that water (brine) confined in the crust of Mars was mobilized in a convective manner and would pass into the supercritical water zone during the down-going leg (the recharge leg) of the convective cell. The zones with supercritical out-salting would require accommodation space for large masses of solid salt, as modeled in the Red Sea analogy. However, as the accommodation space for the solid salt fills up, it will pile up and force its way upwards to form large, perhaps layered anticlines, as seen in the Hebes Mensa area of Mars and at numerous locations on Earth, including the Red Sea. Thus, we offer a universal ‘hydrothermal salt model’, which would be viable on all planets with free water in their interiors or on their surfaces, including Mars and Earth.
Hovland, M., Rueslåtten, H.G., Johnsen, H.K., Kvamme, B., Kutznetsova, T., 2006. Salt formation by supercritical seawater and submerged boiling. Marine and Petrol. Geol. 23, 855-69
Numerical modeling of a hydrothermal system in the Atlantis II Deep of the Red Sea indicates that a shallow magma-chamber causes a sufficiently high heat-flow to drive a convection cell of seawater. The model shows that salt precipitates along the flow lines within the supercritical region (Hovland et al., 2006). During the various stages of planet Mars’ development, it must be inferred that zones with very high heat-flow also existed there. This meant that water (brine) confined in the crust of Mars was mobilized in a convective manner and would pass into the supercritical water zone during the down-going leg (the recharge leg) of the convective cell. The zones with supercritical out-salting would require accommodation space for large masses of solid salt, as modeled in the Red Sea analogy. However, as the accommodation space for the solid salt fills up, it will pile up and force its way upwards to form large, perhaps layered anticlines, as seen in the Hebes Mensa area of Mars and at numerous locations on Earth, including the Red Sea. Thus, we offer a universal ‘hydrothermal salt model’, which would be viable on all planets with free water in their interiors or on their surfaces, including Mars and Earth.
Hovland, M., Rueslåtten, H.G., Johnsen, H.K., Kvamme, B., Kutznetsova, T., 2006. Salt formation by supercritical seawater and submerged boiling. Marine and Petrol. Geol. 23, 855-69
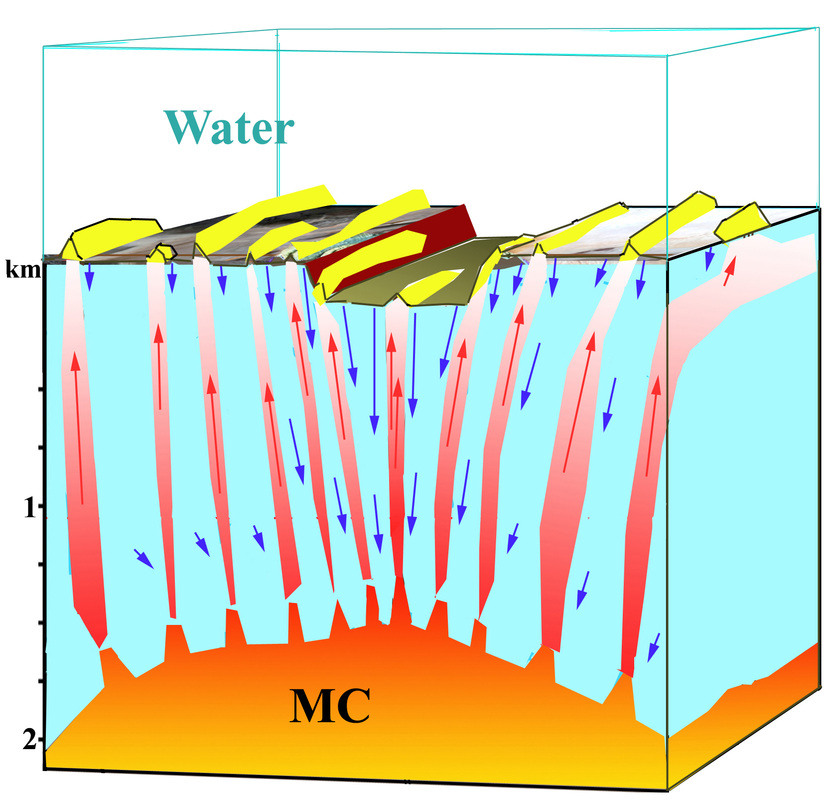
Salt Cauldrons in the Red Sea
The Red Sea is the modern analogy of what happened to the Atlantic Ocean about 112 million years ago. It is a modern rifting ocean, where seawater comes very close to the magmachamber ("MC" in the above sketch). Here the water becomes supercritical and most of the salt is precipitated subsurface. Because there is no room for salt in the sub-surface, the solid salt particles flow upwards towards the surface, where it either flows out onto the seafloor or builds great salt domes and ridges (yellow hills on the sketch). These are salt domes (previously called salt diapirs). Such structures also occur on Mars, for example Hebes Mensa. Such salt domes can also pipe internal flows of water and petroleum.
See our article here: http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2117.2006.00290.x/abstract;jsessionid=12C5F2B88630441A2E8B3EFBFC6BFB1E.d01t02?deniedAccessCustomisedMessage=&userIsAuthenticated=false
See our article here: http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2117.2006.00290.x/abstract;jsessionid=12C5F2B88630441A2E8B3EFBFC6BFB1E.d01t02?deniedAccessCustomisedMessage=&userIsAuthenticated=false
Salt on Mars
Salt is also abundant on Mars - here are some Google Mars images that show salt-features on Mars. Following these image are earth equivalents, from Google Eart:
http://seepology.weebly.com/mars-images.html
http://seepology.weebly.com/mars-images.html